1. What Is CRISPR/Cas9 Gene Editing?
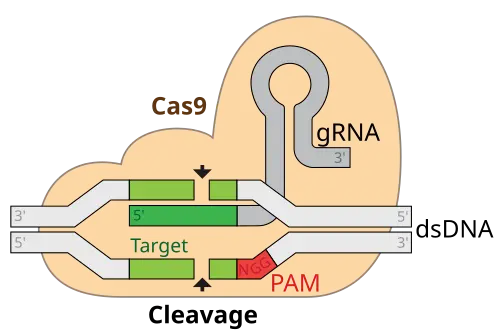
CRISPR/Cas9 is a revolutionary gene-editing technology that gives scientists the ability to make highly specific changes to DNA in living organisms. It is derived from a natural defense mechanism found in bacteria, where they use CRISPR sequences and the Cas9 enzyme to identify and destroy invading viruses. In the lab, scientists have repurposed this system to target and modify any gene of interest by designing a custom guide RNA that directs Cas9 to the desired DNA sequence.
Once the Cas9 enzyme finds the target, it cuts the DNA at that exact spot. This allows scientists to delete a faulty gene, insert a corrected version, or even switch off harmful genetic instructions. What makes CRISPR/Cas9 particularly powerful is its precision, low cost, and flexibility. Unlike earlier gene-editing tools, it doesn’t require complex protein engineering and works in almost any cell type—including human neurons and blood vessels. Today, it is widely used in genetics research and emerging areas of medicine.
2. CRISPR’s Role in Treating Cerebrovascular Diseases
Cerebrovascular diseases involve problems in the brain's blood vessels and include conditions such as stroke, cerebral aneurysms, and vascular malformations. These disorders can be caused or worsened by genetic mutations that affect how blood vessels grow, function, and respond to stress. CRISPR/Cas9 is being explored as a therapeutic solution to address these underlying causes, offering the potential for long-term prevention rather than temporary symptom relief.
One promising target is the NOTCH3 gene, mutations of which cause CADASIL—a rare but serious genetic condition that leads to repeated strokes and dementia. By using CRISPR to correct this mutation, researchers are working toward therapies that could halt disease progression early. In more common cerebrovascular conditions, such as hypertension-induced stroke, scientists are studying genes involved in vascular tone, elasticity, and inflammation, such as ACE and AGT. Editing these genes could potentially help prevent the vessel narrowing or rupturing that triggers stroke events.
Furthermore, CRISPR can be used to repair or stabilize endothelial cells (the cells lining blood vessels) to reduce plaque formation and strengthen the vascular wall, helping to prevent aneurysms and improve blood-brain barrier function. While still experimental, these approaches could dramatically reduce the global burden of stroke and other brain-related vascular events.
3. CRISPR and Neurodegenerative Diseases: Hope for Alzheimer’s
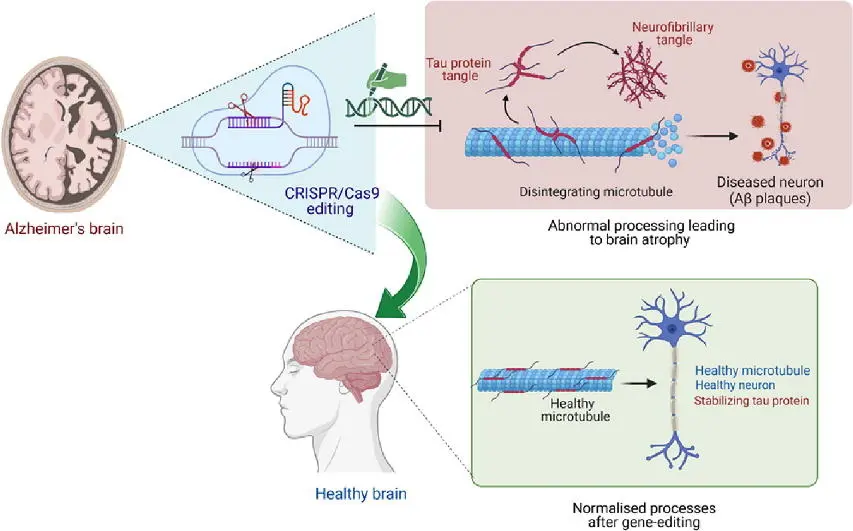
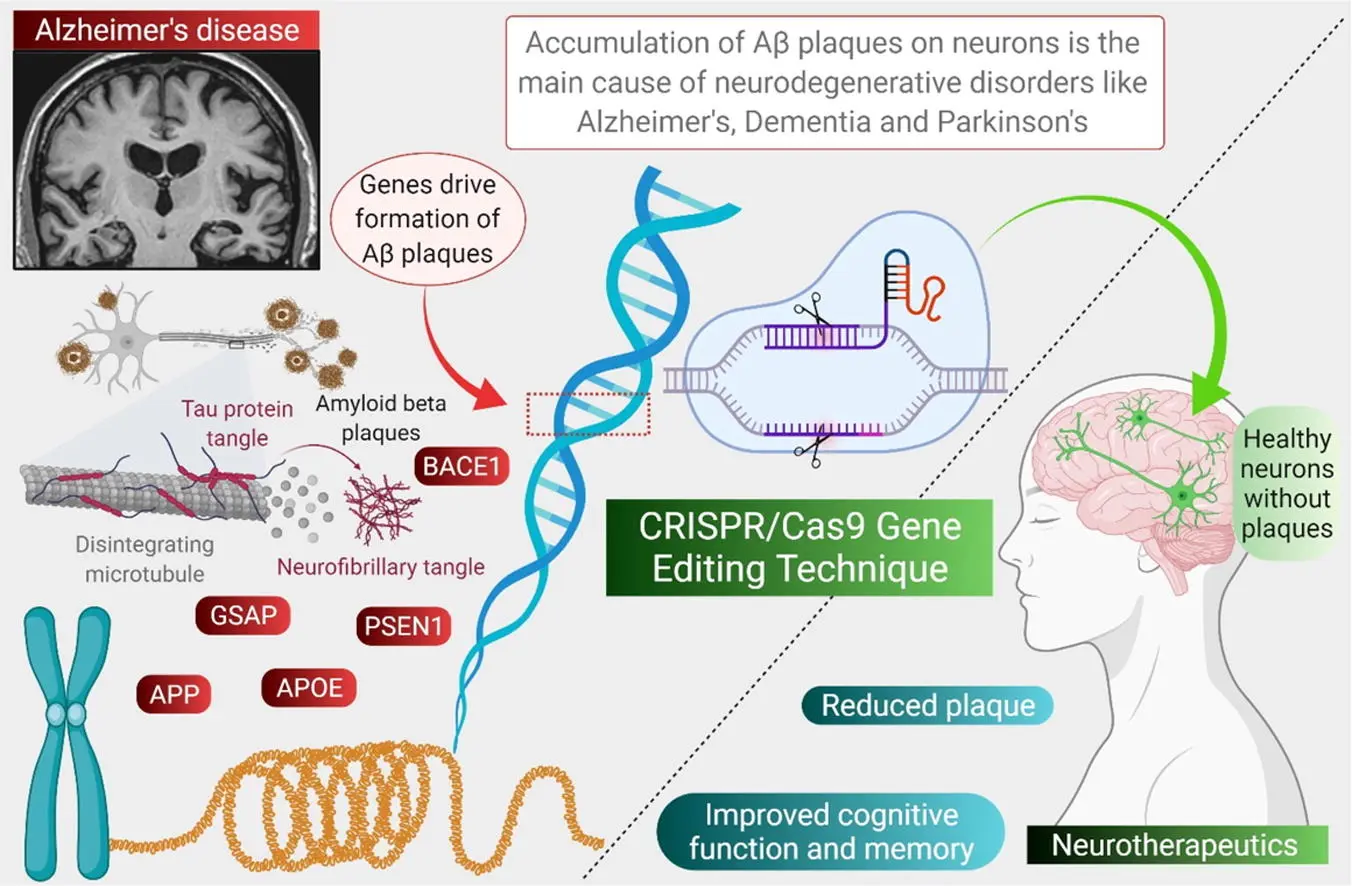
Alzheimer’s disease is a devastating neurological condition characterized by memory loss, confusion, and behavioral decline. It is linked to both genetic and environmental factors. Several genes are known to increase the risk of developing Alzheimer’s, such as APOE4, APP, PSEN1, and PSEN2. CRISPR is being actively researched as a way to correct or silence these genes before they trigger harmful changes in the brain.
For example, APOE4 is a variant of the APOE gene that is strongly associated with a higher risk of Alzheimer’s. Researchers have already used CRISPR to convert the harmful APOE4 variant into the safer APOE3 version in human brain cells. This genetic change resulted in reduced inflammation, less protein aggregation, and improved cell survival. In addition, CRISPR has been used to reduce or knock out the activity of the APP gene and its associated enzymes, which are involved in the production of amyloid-beta plaques—a hallmark of Alzheimer’s.
In animal models, these genetic interventions have led to better cognitive function, reduced brain damage, and even reversal of some Alzheimer’s-like symptoms. Though clinical trials in humans are still at early stages, the future looks promising. CRISPR may one day be used to prevent Alzheimer’s in genetically at-risk individuals, offering hope where conventional drugs have largely failed.
4. Delivery Systems: Overcoming the Blood-Brain Barrier
One of the biggest technical challenges in applying CRISPR to the brain is getting the gene-editing system into the correct cells. The blood-brain barrier (BBB) is a protective shield that prevents most substances, including drugs and large molecules, from entering the brain from the bloodstream. This is beneficial for blocking toxins, but it also makes gene therapy difficult.
To overcome this, researchers are developing innovative delivery methods. One popular approach is using AAV (Adeno-Associated Viruses)—small, non-pathogenic viruses engineered to deliver CRISPR components into neurons or endothelial cells. These vectors are safe and can be targeted to specific areas of the brain. Another exciting technology is Lipid Nanoparticles (LNPs), which were successfully used in COVID-19 mRNA vaccines. LNPs can carry CRISPR RNA and Cas9 protein across the BBB when injected systemically or intranasally.
Other emerging techniques include magnetic nanoparticles that can be guided to precise brain regions using external magnets, and self-assembling nanogels that release CRISPR payloads in response to brain-specific signals. Together, these technologies aim to ensure safe, efficient, and targeted gene editing inside the brain—making clinical treatment with CRISPR more feasible than ever before.
5. Examples of CRISPR in Action
Recent studies have demonstrated CRISPR’s powerful potential in living systems. In one experiment, researchers used CRISPR to correct the APP gene mutation in a mouse model of Alzheimer’s. The treated mice showed improved learning and memory and had fewer plaques in their brains. Another study successfully used CRISPR to reduce the expression of inflammatory genes in blood vessel cells, preventing stroke-like damage in brain tissue.
In the vascular field, scientists have edited genes responsible for excessive blood clotting, which is a major cause of ischemic stroke. By silencing genes like F11 and SERPINE1, CRISPR-based therapies have reduced clot formation in animal models, opening up new avenues for preventing strokes without the need for blood thinners.
Biotech companies such as Editas Medicine, Intellia Therapeutics, and CRISPR Therapeutics are already running clinical trials for CRISPR-based treatments of rare genetic diseases, and many of their technologies are adaptable to brain applications. Research institutions like the Broad Institute and Stanford University are also leading efforts to develop brain-targeted gene-editing therapies.
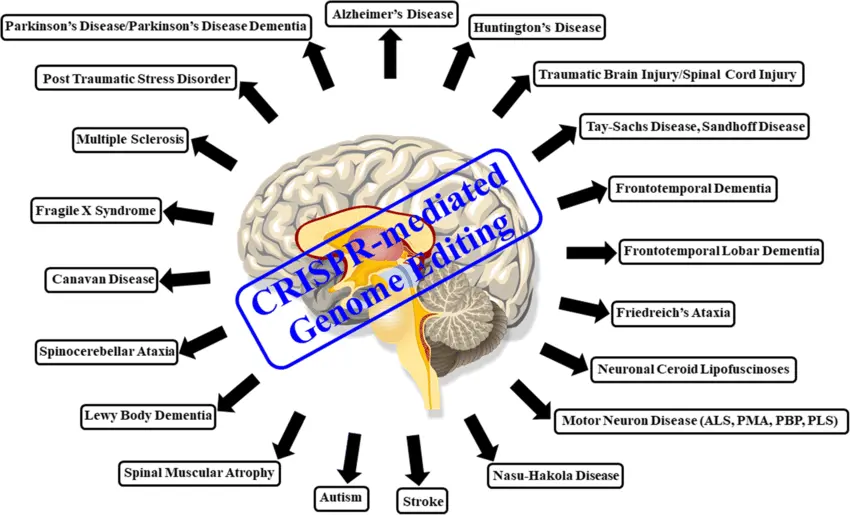
6. The Future of CRISPR in Brain and Vascular Medicine
The dream of preventing neurological and cerebrovascular diseases at the genetic level is no longer science fiction. CRISPR/Cas9 is ushering in a new era of precision medicine—one where doctors could correct disease-causing mutations in a person’s DNA before symptoms ever appear. For complex diseases like stroke and Alzheimer’s, which involve many risk factors, CRISPR could be used in combination with genetic screening, AI-based diagnostics, and biomarker analysis to create highly personalized treatment plans.
Future possibilities include in utero gene correction for inherited brain conditions, CRISPR-equipped neural implants that monitor and repair brain tissue in real time, and injectable CRISPR therapies that rejuvenate aging neurons. As safety improves and delivery becomes more efficient, CRISPR is poised to become a central pillar of brain and vascular healthcare.
Of course, ethical questions and regulatory challenges remain. But with growing public support, academic breakthroughs, and industry investment, the path toward safe, effective, and accessible CRISPR therapies is being paved today.
Further Reading
To explore this topic in more depth, you can read this peer-reviewed article:
CRISPR/Cas9 gene editing: New hope for Alzheimer's disease therapeutics